Q.
A solid AB crystallises in $NaCl$ structure. The anion $B ^{-}$form fcc lattice, while the cation $A ^{+}$ occupy all the octahedral holes. If all the particles as shown in the figure are removed, then the formula of solid becomes :-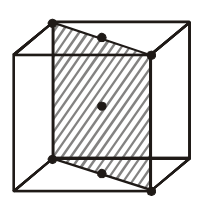
The Solid State
Solution: