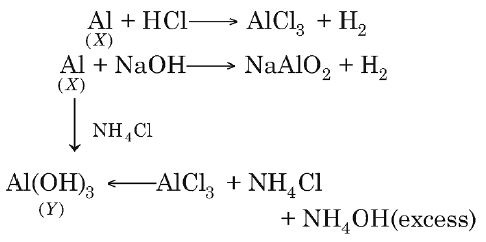Q. A metal $(X)$ dissolves both in dil. $HCl$ and dil. $NaOH$ to liberate $H_{2}$. Addition of $NH_{4}Cl$ and excess $NH_{4}OH$ to an $HCl$ solution of $X$ produces $Y$ as a precipitate. $Y$ is also produced by adding $NH_{4}Cl$ to the $NaOH$ solution of $X.$ The species $X$ and $Y,$ respectively, are
KVPYKVPY 2015
Solution: