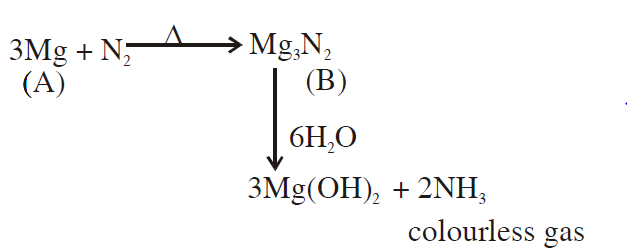Q. A metal (A) on heating in nitrogen gas gives compound $B. B$ on treatment with $H_2O$ gives a colourless gas which when passed through $CuSO_4$ solution gives a dark blue-violet coloured solution. $A$ and $B$ respectively, are :
Solution:
$CuSO_4 + 4NH_3 \rightarrow \underset{\text{deep blue solution}}{[Cu(NH_3)_4]SO_4}$