Q.
A mass of an ideal gas of volume V at pressure P undergoes the cycle of changes shown in the graph
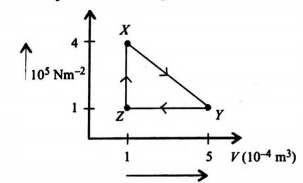
At which points is the gas coolest and hottest?
coolest
hottest
(a)
X
Y
(b)
Y
X
(c)
Y
Z
(d)
Z
Y
| coolest | hottest | |
| (a) | X | Y |
| (b) | Y | X |
| (c) | Y | Z |
| (d) | Z | Y |
Thermodynamics
Solution: