Q.
$A$ gas can be taken from $A$ to $B$ via two different processes $ACB$ and $ADB$.
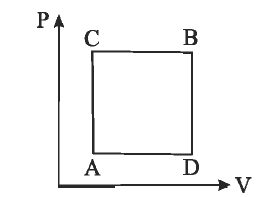
When path $ACB$ is used $60 J$ of heat flows into the system and $30J$ of work is done by the system. If path $ADB$ is used work done by the system is $10 J$. The heat (in joule) flow into the system in path $ADB$ is :
Thermodynamics
Solution:
