Q.
A fixed mass $\text{m}$ of a gas is subjected to transformation of states from $\text{K}$ to $\text{L}$ to $\text{M}$ to $\text{N}$ and back to $\text{K}$ as shown in the figure.
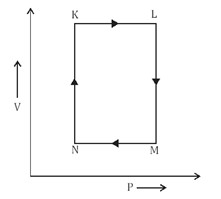
The succeeding operation that enable this transformation of state are
NTA AbhyasNTA Abhyas 2020Thermodynamics
Solution: