Q.
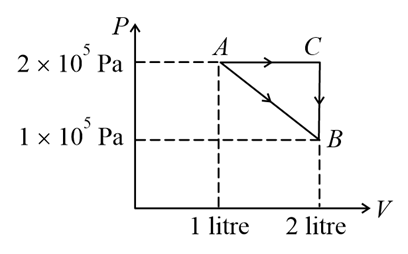
A certain substance (not an ideal gas) has a $PV$ graph as a straight line $AB$ when subjected to an adiabatic process. If the same substance goes through process $A \rightarrow C \rightarrow B,$ what is the heat given to it?
NTA AbhyasNTA Abhyas 2022
Solution: