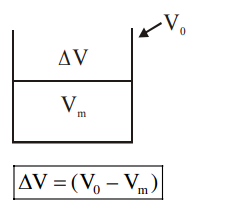
Q. A bakelite beaker has volume capacity of $500 cc$ at $30^{\circ} C$. When it is partially filled with $V _{ m }$ volume (at $30^{\circ}$ ) of mercury, it is found that the unfilled volume of the beaker remains constant as temperature is varied. If $\gamma_{\text {(beaker) }}=6 \times 10^{-6}{ }^{\circ} C ^{-1}$ and $\gamma_{(\text {mercury })}=1.5 \times 10^{-4}{ }^{\circ} C ^{-1},$ where $\gamma$ is the coefficient of volume expansion, then $V _{ m }$ (in $cc$ ) is close to________.
Solution: