Q.
$A_1$ and $A_2$ are two ores of metal $M.A_1$ on calcination gives black precipitate, $CO_2$ and water.
Identify $A_1$ and $A_2$.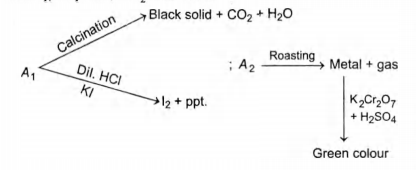
IIT JEEIIT JEE 2004General Principles and Processes of Isolation of Elements
Solution: