Q. 5.6 g of an organic compound on burning with excess of oxygen gave 17.6 g of $ C{{O}_{2}} $ and 7.2 g of $ {{H}_{2}}O $ . The organic compound is:
KEAMKEAM 2006
Solution:
$ Organic\text{ }compound\xrightarrow{[O]}\underset{17.6\,g}{\mathop{C{{O}_{2}}}}\,+\underset{7.2g}{\mathop{{{H}_{2}}O}}\, $
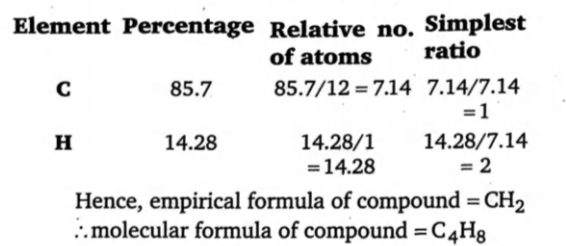
% of $ C=\frac{12}{44}\times \frac{17.6}{5.6}\times 100=85.7 $ %
% of $ H=\frac{2}{18}\times \frac{7.2}{5.6}\times 100=14.28 $ %