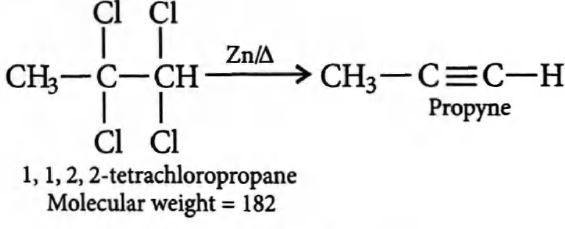
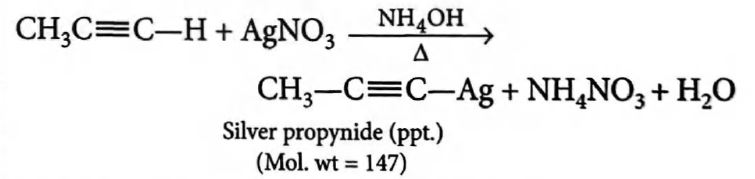
Q. $36.4 \,g$ of $1$, $1$, $2$, $2$-tetrachloropropane was heated with zinc dust and the product was bubbled through ammonical $AgNO_3$. What is the weight of precipitate obtained?
Haloalkanes and Haloarenes
Solution: