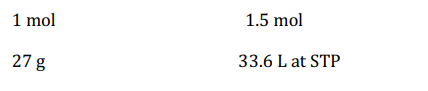Q. $3.9\, g$ of a mixture of aluminium and its oxide on reaction with aqueous solution of sodium hydroxide, gave $840 \,mL$ of a gas under standard conditions. Thus, aluminium content in the mixture is
Some Basic Concepts of Chemistry
Solution: