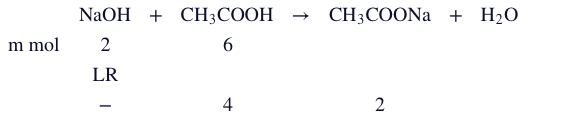
Q. $20 \, ml$ of $0.1 \, M \, NaOH$ is added to $30 \text{ ml} 0.1 \text{ M NaOH } 30 \text{ ml} \, 0.2 \left(\text{ M CH}\right)_{3} \text{COOH } \left(\left(\text{pK}\right)_{\text{a}} = 4.74\right)$ , calculate the $\text{pH}$ of the resulting solution? [given: $log 2=0.3$ ]
NTA AbhyasNTA Abhyas 2020Equilibrium
Solution: