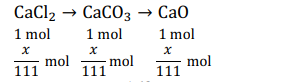Q. $10 \,g$ of a sample of a mixture of $CaCl _2$ and $NaCl$ is treated to precipitate all the calcium as $CaCO _3$. This $CaCO _3$ is heated to convert all the $Ca$ to $CaO$ and the final mass of $CaO$ is $1.62 \,g$. The precent by mass of $CaCl _2$ in the original mixture is
Some Basic Concepts of Chemistry
Solution: