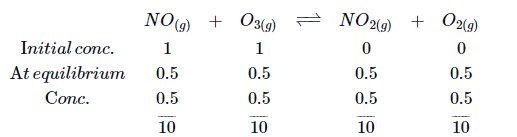Q.
$1$ mole of $NO$ and $1$ mole of $O_3$ are taken in a $10\,L$ vessel and heated. At equilibrium, $50\%$ of $NO$ (by mass) reacts with $O_3$ according to the equation:
$NO_{(g)} +O_{3(g)} \rightleftharpoons NO_{2(g)} +O_{2(g)}$.
What will be the equilibrium constant for this reaction?
Equilibrium
Solution: